Tocco Report: EU PPWR: The Definitive Implementation Guide 2026-2040
Recyclability, recycled content, reuse, & PFAS-free rules from 2026 to 2040
The Packaging and Packaging Waste Regulation (PPWR) is not another incremental tweak to Brussels paperwork. It is indeed a hard reset of how packaging is designed, sourced, shipped, collected, and audited in Europe – and, by extension, for any supplier that wants access to the EU market.
At Tocco Editorial, we read it as the moment where recyclability, reuse performance, chemical safety, and data transparency stop being “nice to have” and become conditions for market entry.
This report is structured as a practical implementation guide rather than a legal commentary. It distils the final legislative text, technical annexes, and early industry responses into 5 operational pillars: recyclability grading, recycled content, reuse, waste minimisation, and chemical safety. Our aim is simple: give decision-makers a clear view of what changes between now, 2030, and 2040 – and the detail they need to act in order.

Explore This Report
Start with the Free Version or unlock the Full Report with deeper insights, case studies, and extra content.
- The Regulatory Architecture and Strategic Timeline
- The Recyclability Performance Grading System (A-E)
- The Recycled Content Mandate and Supply Chain Resilience
- The Reuse Revolution & Reverse Logistics
- Waste Minimization and Annex V Bans
- Chemical Safety and the De Facto PFAS Ban
- Digital Compliance: The Digital Product Passport (DPP)
- Sector-Specific Impact and Strategic Outlook
- Strategic Implementation Roadmap: A4-Step Strategy

Materials in focus
Explore the innovative materials shaping the future

Biodegradable

Recycled

Bamboo

Mycelium

Silk

Upcycled

Cotton

Linen

Latex

Bioplastic

Hemp

Leather

Jute

Banana Fibre

Wool

Fur

Sisal
What's inside?
 something
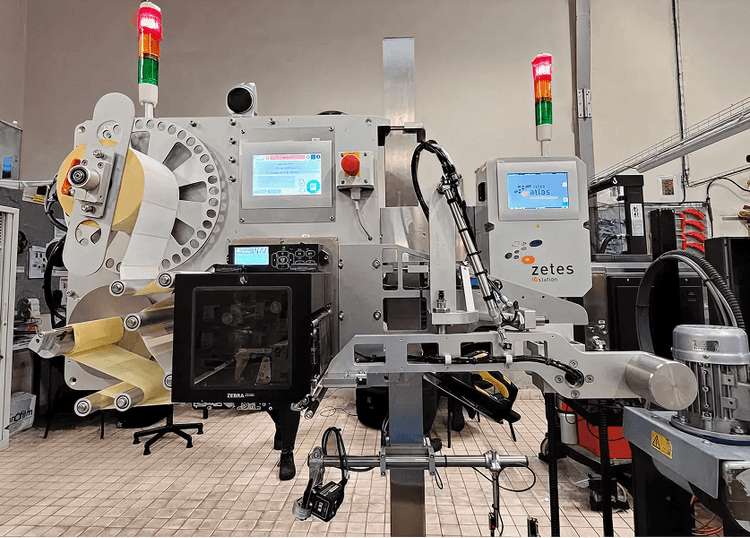
somethingZetes - ZetesAtlas
ZetesAtlas, a packaging execution system that generates, prints and camera-verifies unique identifiers at high line speeds. Its track record in EU Falsified Medicines Directive serialisation proves the model: unit-level IDs aggregated across cases and pallets, with a clean audit trail back to the line. The same stack is now being redeployed for consumer packaging.
Other Available Reports
Expand your knowledge with our curated collection of industry-leading insights
Latest Design Stories
Weekly highlights from our community of innovators and pioneers